Abstract
Summary of Work
The amino acid sequence of the M-protein for multiple myeloma (MM) is unique compared to the polyclonal antibodies in patients' blood. In this study we utilize this uniqueness to develop an ultra-sensitive M-protein detection method with mass spectrometry (MS). The method involves the de novo sequencing of the amino acid sequence of the M-protein from the baseline blood sample collected at the time of diagnosis, and a targeted MS assay to detect and quantify the unique M-protein sequence in the follow-up blood samples. This non-invasive method is purely blood based and is 3,000 and 300 times more sensitive than SPEP and IFE, respectively.
De Novo Protein Sequencing
The M-protein sequencing is carried out on an MS based platform (REmAb). The effectiveness of de novo protein sequencing has been proven with the sequencing of hundreds of monoclonal antibodies (McDonald et al., Poster 294737, ASMS, 2018). In the current study the M-protein from 4 MM patients' baseline blood samples were successfully sequenced, demonstrating robustness of the method in the presence of the polyclonal background. As little as 50ul of the serum was required for the de novo sequencing.
Sensitivity of M-protein Quantification
The lower limit of quantification (LLOQ) was studied with a serial dilution experiment. The baseline blood sample of one patient was sequentially diluted with a healthy donor's serum. The peptide sequence unique to the M-protein was monitored with mass spectrometry to detect and quantify the M-protein in the serial dilution. The M-protein could still be detected and quantified when the dilution ratio was 1:10,000 (the amount of M-protein relative to background polyclonal serum IgG). In a separate experiment, synthesized heavy labelled proteotypic peptides were used to estimate the LLOQ in IgG enriched serum at 60 ug/dL, over 3,000 times more sensitive than SPEP (0.2g/dL) (Bergen et al., Clin Chem 62: 1 243-251, 2016) and 300 times more sensitive than IFE (0.02g/dL) (IMWG, British Journal of Haematology, 121, 749-757, 2003). As little as 30ul of serum was required in these experiments for monitoring of M-protein levels.
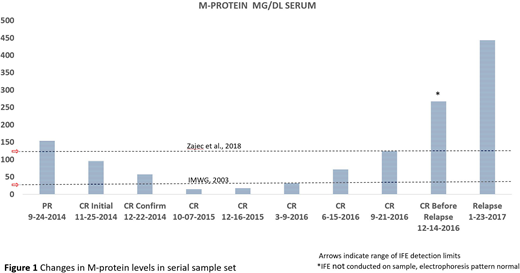
Case Study
A targeted MS based assay was developed to monitor the M-protein levels of a serial patient sample set (73-year-old male, treatment: Elotuzumab/Lenalidomide/Dexamethasone, progression free survival 32 months). M-protein levels were quantified in a total of 10 serial samples from partial remission, through complete remission (CR), until relapse over a period of 2 years and 3 months (Figure 1). M-protein could be detected and quantified in all samples collected during the CR period with estimated M-protein levels never below 10mg/dL. Notably, a 2-fold increase in M-protein levels (in comparison to the lowest historic level) could be detected 320 days prior to the timepoint of relapse. The upward trend continued in the next 3 serial CR samples preceding relapse. Except for one sample (see Figure 1, 'CR Before Relapse 12-14-2016'), CR was based on the absence of M-protein in the IFE result in the relevant clinical data.
Conclusion
The work represents a first step in the application of de novo sequencing and MS based detection for the sensitive monitoring of M-protein levels in serum. It shows a much-improved sensitivity over current standard approaches and has the great potential to provide non-invasive assessment of MRD for multiple myeloma. The preliminary data warrant further development of this MS based non-invasive and highly sensitive M-protein quantification method.
McDonald:Rapid Novor Inc: Employment, Equity Ownership. Liu:Rapid Novor Inc: Employment, Equity Ownership. Taylor:Rapid Novor Inc: Employment, Equity Ownership. Yang:Rapid Novor Inc: Employment, Equity Ownership. Ma:Rapid Novor Inc: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal